This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Digital Microscopy-based Malaria Diagnostic Solution,
miLab™ MAL
miLab MAL was introduced in UNITAID's report in 2022 as
an innovative product that overcomes the limitations of current
malaria diagnosis.

The Most Advanced Digital Microscope and
Fully Integrated Bench-top Platform
miLab™ MAL, the malaria diagnostic solution is getting attention from the international community as an innovative malaria diagnostic product that overcomes the limitation of rapid diagnosis testing and manual microscopic diagnosis. miLab™ MAL was introduced in UNITAID’s annual report* as an innovative product that will enter scale-up level.
- * Malaria Diagnostics Market and Technology Landscape, 4th
- Download
- World Malaria Report 2022
- Download
miLab™ MAL focuses on overcoming the limitations of traditional diagnostics
for malaria elimination in the world.
Malaria is one of the deadliest infectious diseases that international society, including the UN and WHO, announced will end the
epidemics by 2030.
Global Malaria Issue Overview
* Year 2021
Key Issues of Malaria Diagnosis and Limitations of Current Methods of Diagnosis
Microscopy Test There are insufficient opportunities for qualified microscopy testing due to the cost of maintaining infrastructure and experts. HRP 2/3 Deletion There are problems with RDT diagnostic performance because of the emergence of HRP (Histidine-rich protein 2) gene deletion species. Malaria Imported Cases As cross-border travel increases, the risk of contact with various malaria species regardless of the region also increases. Drug Resistance The need for periodic monitoring of the effectiveness of malaria treatments increases due to the spread of drug resistant strains of malaria.
The World’s Only Diagnostic Testing Solution with the Accuracy of a Diagnostic
Laboratory and the Accessibility of Rapid Diagnostic Tests
- Performs Qualified Microscopy Test without Investment on Additional Infrastructure and Experts
- Based on proven AI technology, miLab™ MAL performs accurate diagnostic tests at the level of qualified microscopists. In addition, it is easy to use the miLab™ with only about two hours of training. miLab™ Viewer allows the experts to review with remote access if an additional review is necessary.
- Morphological Diagnosis Method, Independent of HRP 2/3 gene deletion Issue
- Unlike RDT that determine infection by antigen-antibody reactions, accurate diagnostic tests are possible regardless of HRP 2/3 gene deletion issues that are recently spreading around Africa.
- Support of In-depth Analysis of Malaria Parasite Species Pre-Classification and Life Cycle Stage
- Through AI morphology analysis, miLab™ allows users to check the pre-classification of species and life cycle stage information. Based on this, it is possible to provide accurate treatment and monitoring of malaria-infected patients.
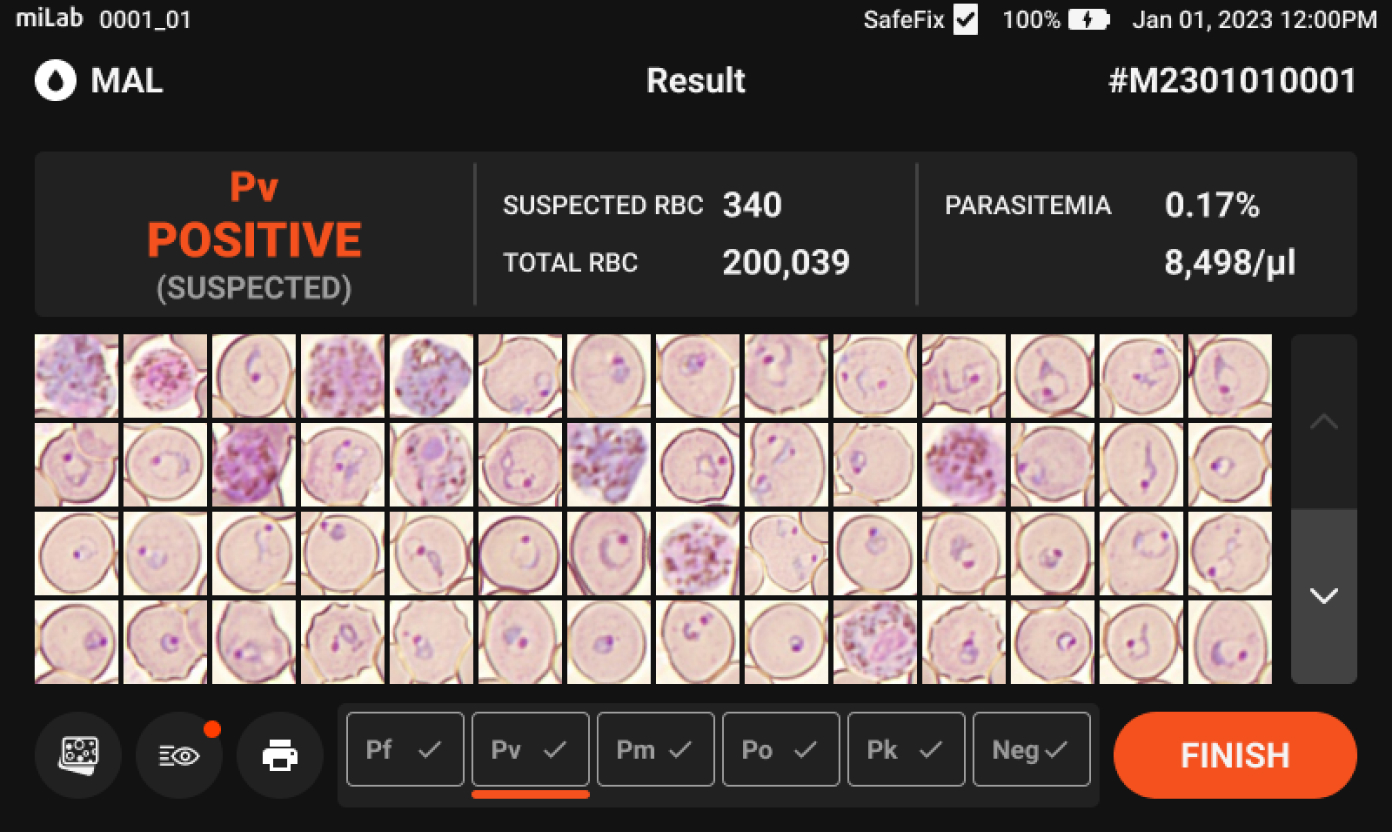
- Provides Quantitative Parasitemia Numerical Analysis
- Through analysis of 100K~300K red blood cells, which is more than 100 times more than the number checked by microscopists, it is possible to provide a more reliable parasitemia level estimate.
miLab™ MAL Delivers Differentiated Value in Terms of Improved Accessibility and Workflow Improvements Based on the User’s Needs.
- 1. Regions with a High Proportion of Imported Malaria Infections (North America, Europe, Middle East, etc.)
-
- Target customer
- National malaria diagnostic laboratory in the countries with no indigenous malaria infection.
- Expected Value
- Improved workflow allows lower labor costs and higher operational efficiency
- Background
- In high income countries like the United States, there is not much demand for malaria diagnostic testing because there are no Indigenous malaria infections. Nevertheless, malaria is an infectious disease managed at the national level and national diagnostic laboratories must have malaria diagnostic testing capabilities. In addition, since cross-border travel is increasing as the world recovers from COVID-19, malaria infections risks are also growing.
- Problem
- Diagnostic labs need to hire high quality microscopists, but only a few have expertise and abundant experience in diagnosing malaria due to the low demand for the tests. In addition, labor costs for diagnostic testing have risen rapidly worldwide due to COVID-19, and the burden of hiring experts in areas where diagnostic test demand is low has increased. In fact, in high-income countries, the number of malaria diagnostic professionals is gradually decreasing.
- Use-Case
- The diagnostic laboratory experiencing difficulties in securing and hiring malaria experts, can secure malaria diagnostic test capabilities by introducing miLab™ MAL. Even without malaria experts, they can easily ask the malaria experts of another diagnostic laboratory to remotely review the digital image captured by the miLab™ platform. In diagnostic laboratories with malaria experts, introducing miLab™ MAL allows them to diagnose with high operational efficiency. This is because the miLab™ platform automatically performs all processes from sample prep, digital imaging, and AI analysis for malaria diagnosis.
- Benefits
- Customers can expect to improve their workflow with the introduction of miLab™ MAL, which reduces labor costs and improves efficiency.
- 2. Regions with a High Proportion of Indigenous Malaria Infections. (Africa and Latin America)
-
- Target customer
- Diagnostic laboratory conducting malaria diagnostic tests in low and middle-income countries with a high incidence of malaria.
- Expected Value
- Increased reliability of diagnostic results by improved workflow.
- Background
- The quality of the microscopic test, a gold standard method for malaria diagnosis, is affected by the proficiency of the experts. This is because the microscopic test is performed manually over eight or more complex steps, including blood smear, staining, and washing. Therefore, in order to maintain the quality of the microscopic test consistently, it is essential to secure experts with the experience and skills to perform it professionally.
- Problem
- In most African countries, where malaria cases are high, it is not easy to secure experts with abundant experience and accurate expertise in microscopic tests. This is because they cannot afford to have the facilities and infrastructures to systematically train experts. Many diagnostic laboratories in Africa are conducting microscopic tests; they are having difficulty securing stable quality, with large variations in test results depending on the proficiency of the microscopists. In particular, the deviation of the test results is actually malaria-positive, but the test results are often found in false negative, since it’s easy to identify the presence of malaria-infected cells, but much harder to confirm the absence of malaria-infected cells.
- Use-Case
- Diagnostic laboratories struggling to manage the quality of malaria diagnostic tests due to inconsistency in the proficiency of experts, can increase the reliability of diagnostic tests by introducing miLab™ MAL. In particular, for the results of malaria-negative reading by experts, they can conduct the second test with miLab™ to verify the results. Additionally, the miLab™ platform can support microscopists’ training with various sample types stored as digital result data.
- Benefits
- miLab™ MAL users can expect to provide more accurate results and treatment to patients by improving the expertise of microscopists and increasing the reliability of the test results.
- 3. Regions with Where pfHRP 2/3 gene deletions Occur
-
- Target customer
- Health facilities that perform malaria diagnostic tests using rapid diagnostic kits in the areas where PfHRP 2/3 genetic mutations occur.
- Expected Value
- Provide accurate diagnostic results even in health facilities with no experts through improved diagnostic accessibility.
- Background
- Most African countries with a large number of malaria cases rely more on rapid diagnostic kits than microscopic diagnostic tests. International public procurement agencies such as the Global Fund are also purchasing rapid diagnostic kits as malaria diagnostic testing tools and distributing them to African countries. The rapid diagnostic kit that has been commercialized so far is checking the presence or absence of Histidine-rich protein 2(HRP2) antibodies that occur when infected with malaria parasites. However, malaria parasites with genetic mutations that do not produce HRP2 antibodies have recently been found in more than half of African countries.
- Problem
- Diagnostic laboratories struggling to manage the quality of malaria diagnostic tests due to inconsistency in the proficiency of experts, can increase the reliability of diagnostic tests by introducing miLab™ MAL. In particular, for the results of malaria-negative reading by experts, they can conduct the second test with miLab™ to verify the results. Additionally, the miLab™ platform can support microscopists’ training with various sample types stored as digital result data.
- Use-Case
- Health facilities located in areas where PfHRP 2/3 genetic mutations are spreading can maintain malaria diagnostic testing services by introducing miLab™ MAL instead of using the unreliable rapid diagnostic kits. miLab™ MAL can be used after some simple training so local healthcare workers who do not have expertise in microscopic diagnosis can easily use it.
- Benefits
- With the introduction of miLab™ MAL, users can expect to provide patients a reliable microscopic diagnostic solution with their existing workforce.
- 4. Regions Where Mixed Infection Cases Occurred (Latin America, Southeast Asia, etc.)
-
- Target customer
- Health facilities that perform malaria diagnostic tests using rapid diagnostic kits in areas where multiple malaria parasite infections are observed.
- Expected Value
- Improved diagnostic accessibility to provide accurate diagnostic test results and to strengthen the surveillance system.
- Background
- In addition to African countries, malaria is also occurring in Latin America and Asia. Malaria parasites found in African countries are mainly P. falciparum, but there are other species such as P. vivax, P. knowlesi, P. ovale, and P. malariae in Latin America and Asia. Since there are differences in biological characteristics depending on malaria parasite species, there are differences in symptoms, diagnosis and treatment for each species. Currently, more than two malaria species are found in more than 30 countries around the world, and other parasite species are reported in countries where only P. falciparum has been previously found.
- Problem
- The rapid diagnosis kit is effective in checking for infection of a single species with a large number of cases, such as P.falciparum and P.vivax, but has the limitation that it cannot be used for mixed infections of two or more species or in the diagnosis of other species. Therefore, in countries where more than two species are found, the antigen antibody based RDT result reliability decreases. In particular, there is a high risk of spread of new malaria parasite species in border areas with large population movement or in dense forest areas where accurate diagnostic tests are not available.
- Use-Case
- In areas where multiple malaria parasite infections are observed, morphological characteristics of parasite species can be confirmed with the introduction of miLab™ MAL in health facilities.
- Benefits
- Customers can expect that the miLab™ MAL will accurately identify the malaria species infecting patients. In addition,new species can be quickly confirmed at the border aiding national infectious disease monitoring of malaria.
- 5. Special Situation
-
Malaria is an infectious disease that occurs widely in 84 countries around the world, and there is a risk of malaria infection in special environments such as military bases, ships, rainforests, conflict zones, and refugee camps. miLab™ MAL is a digital microscope platform that enables accurate and rapid malaria diagnosis even in special situations where infrastructure and professionals are insufficient. We continue to develop our products so that they can be easily used in any environment where malaria diagnosis is needed. Contact us for more information on using miLab™ MAL in special situations.
Check
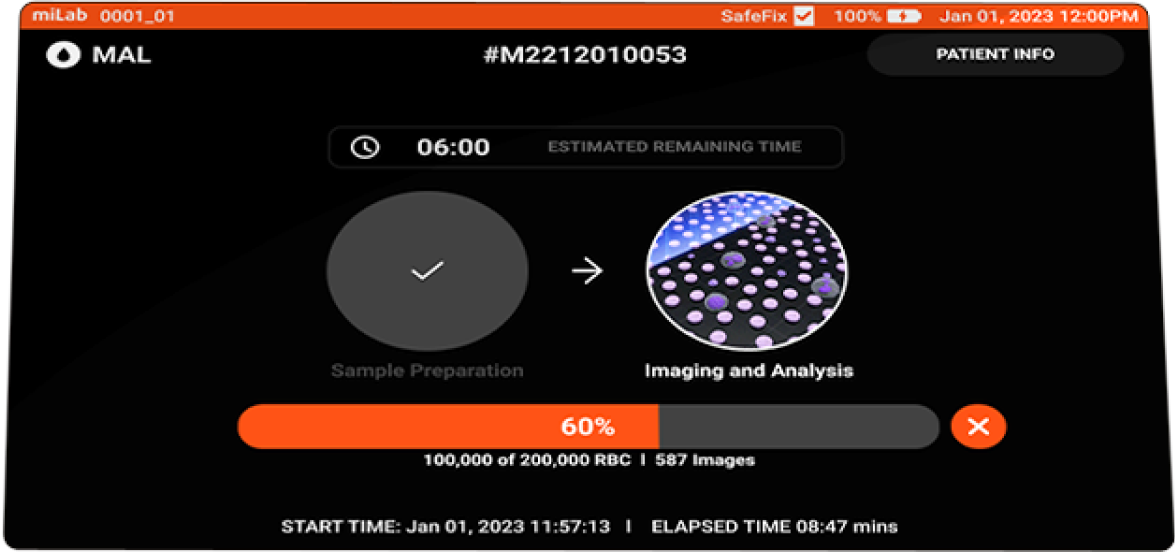
200,000 RBCs
in 15 min.
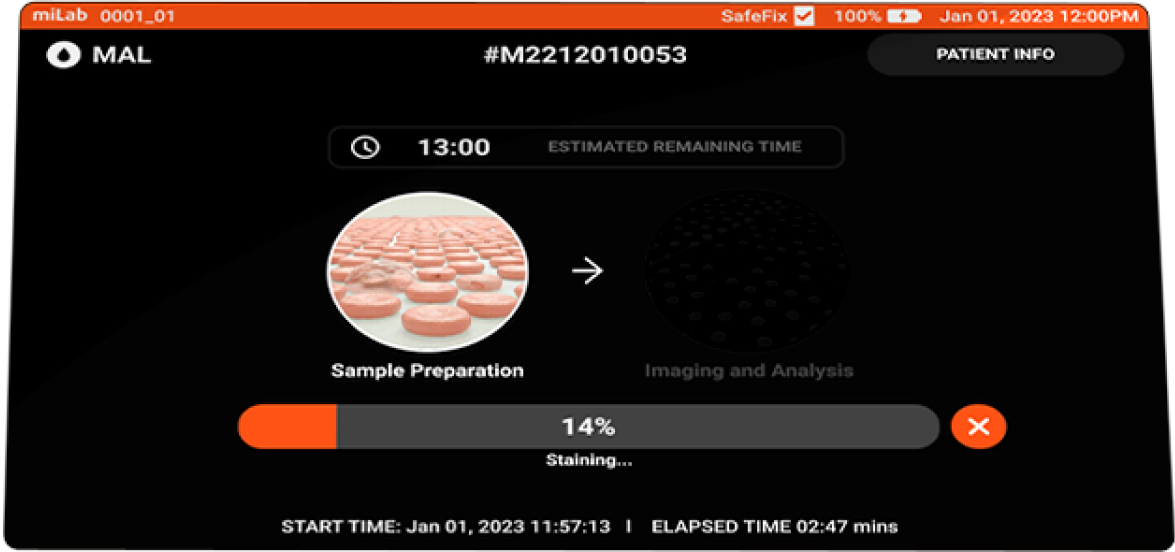
- miLab™ Malaria Specification
-
- Sample Preparation
-
Staining method Modified Romanowsky Sample type Venous or finger-prick blood Sample volume 5μl
- Imaging
-
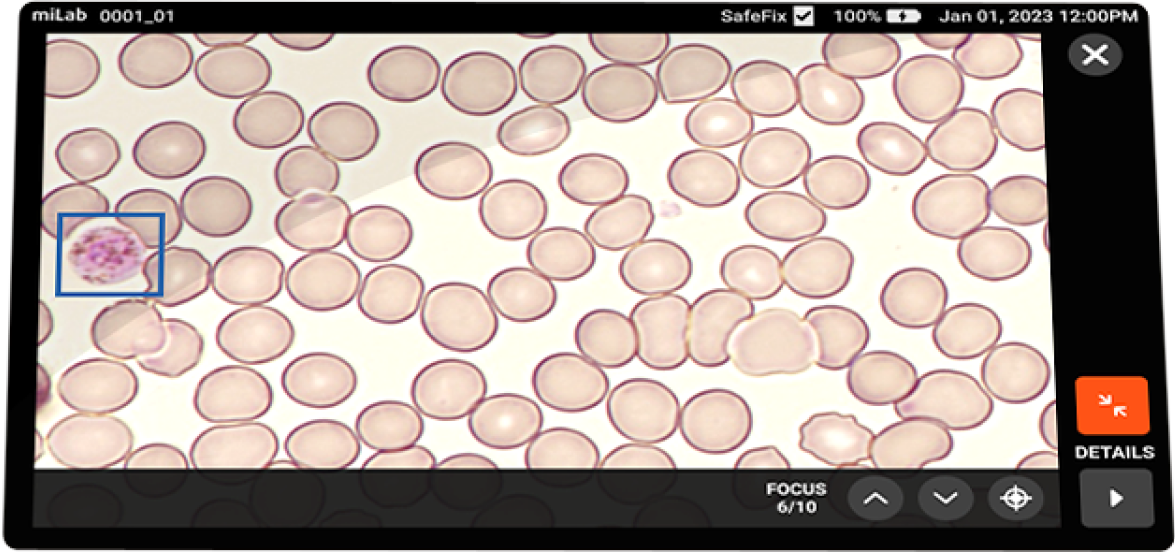
Focusing Multi-focus images acquired Image count Acquires max. of 300,000 RBCS
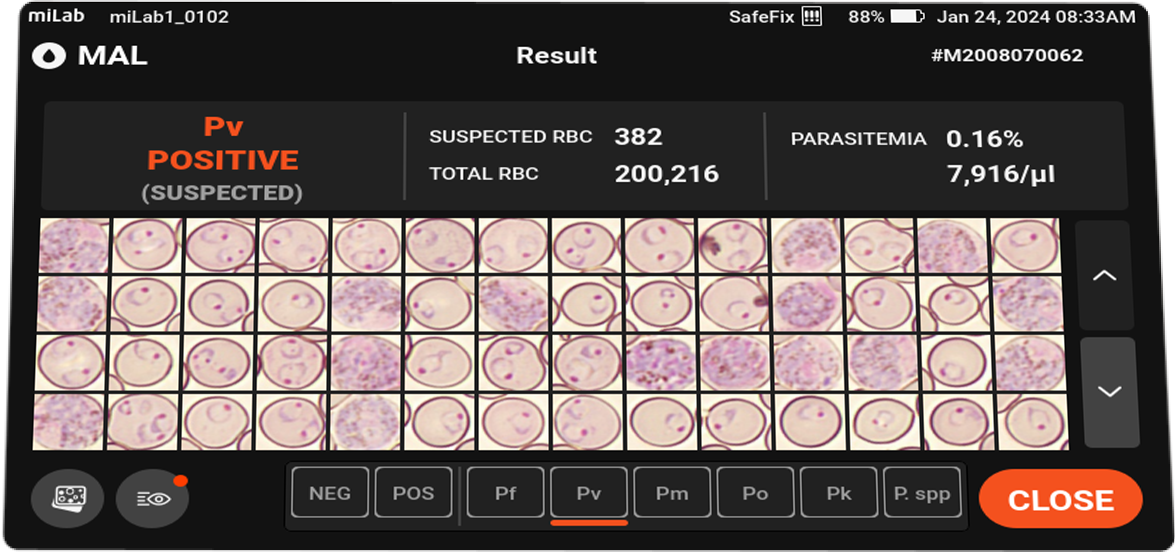
- Analysis
-
Species suggestions Plasmodium falciparum (Pf)
Plasmodium vivax (Pv)
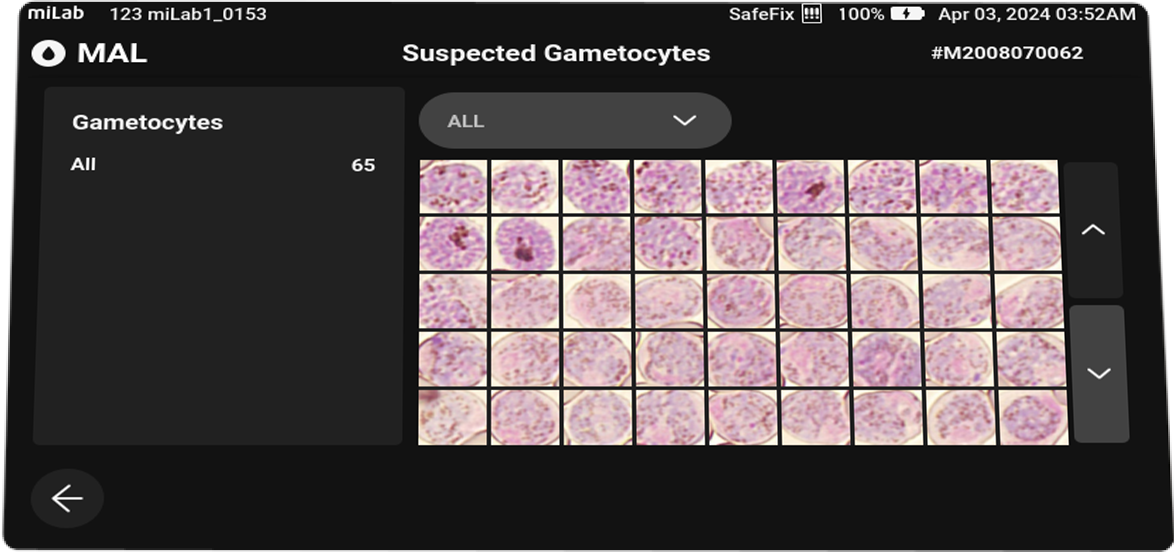
Plasmodium spp. (MP)Stages suggestions Gametocyte, Schizont, Ring / Trophozoite Count Suspected RBC, Total RBC Parasitemia level %, Suspected RBC/μl Viewing Functions Field view
Multi-focus view (10 vertical-planes)
- Total Test Time
-
Avg. 15 mins
- Remote Review
-
Viewing Functions Field View, Cell View, and User Reclassification
- Product Code
-
-
Product Code miLab™ Platform DMLA miLab™ Cartridge MAL CMAA miLab Viewer™ SMVA SafeFix™ CSFA miLab™ Slide Case ASCA
-






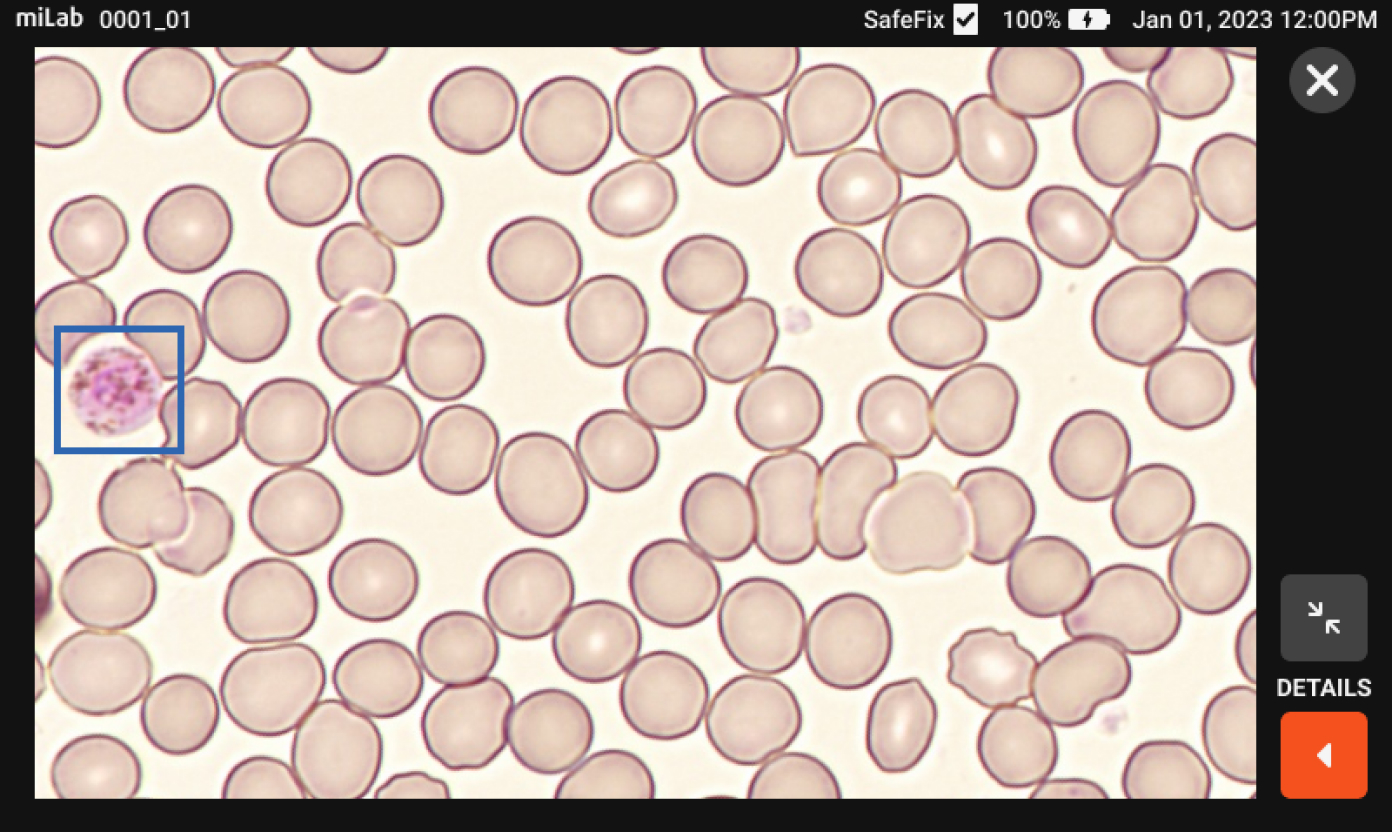
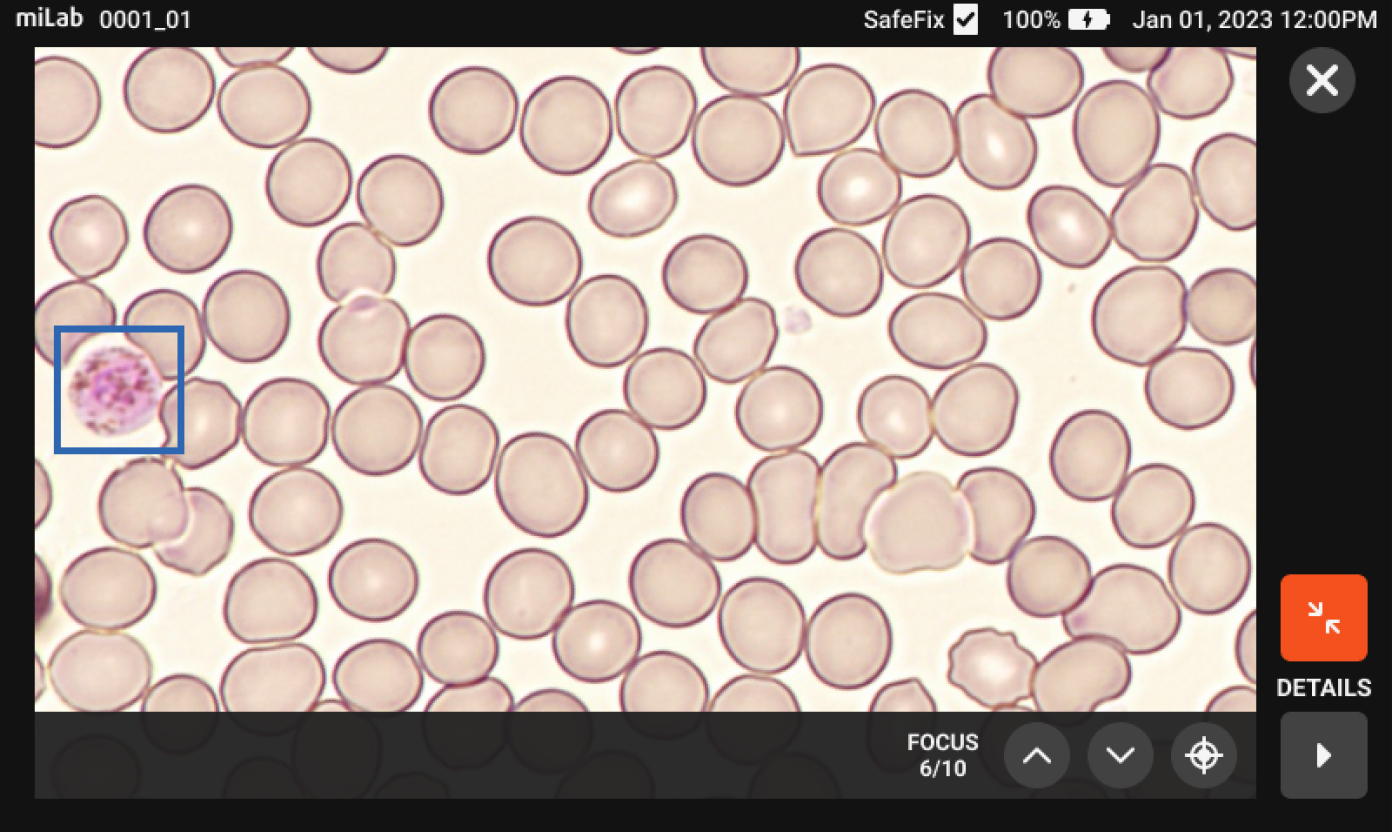

There are many cases of finding malaria-infected patients on the MiLab™ platform that local experts have not found in Ethiopia's clinical sites. Because MiLab™ is easy and simple to use, it's a must-have product in the clinical field
by Cristian Koepfli, Ph.D.
Assistant Professor of University of Notre Dame